Bacterial Cells Often Cannot Produce Recombinant Proteins
Bacteria lack the endoplasmic reticulum and Golgi. C have a high.
Protein Expression in Mammalian Cells.
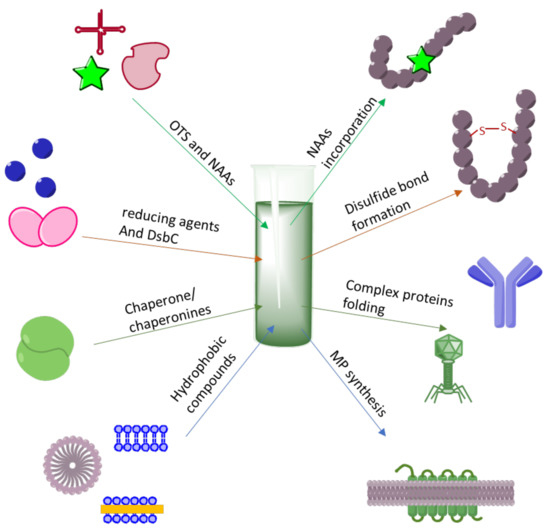
. Thus lower temperatures during induction. 4 What bacteria is commonly used in Rdna technology. As such recombinant protein production has a major role in drug development.
This is done at the genetic level and the two proteins must be in frame to be correctly expressed into protein. The most widely used recombinant expression in bacteria is. Often stable cell lines produce low protein yields and production is not stable over the time.
A central problem with bacteria as hosts for recombinant protein production is their inability to post-translationally modify proteins in the way that human cells can for example glycosylation attachment of antennae of specific sugar epitopes to proteins. The recombinant protein would eventually need to be separated from the carrier protein for further analysis. Coli cells are not glycosylated.
Dumon-Seignovert et al 2004. Coli are 30 kDA and 18 kDA respectively. The reason for this is that bacterial cells.
Future directions in bacterial recombinant protein production are explored. Bacteria did not develop sophisticated mechanisms for performing posttranslational modifications which are present in higher organisms. Coli cells do not possess which organelle.
True 48Yeasts cannot express foreign eukaryotic genes. Recombinant proteins produced by E. Can trigger bacteria to produce inclusion bodies 3.
The choice of the host cell depends on the protein expressed. Bacteria is extensively used to produce recombinant proteins because genetically they are easily manipulated and require only inexpensive components only needed for cultivation Langlais Korn 2006. To culture the bacteria and obtain the protein product the bacteria must grow.
This is because E. Incorrect cellular localization within or outside the cell. Protein overproduction or toxicity.
Smooker2 1Skin Pathogens Research Laboratory Menzies School of Health Research Casuarina NT Australia 2School of Applied Sciences RMIT University. Bacterial hosts are commonly used for the production of recombinant proteins. Bacterial cell such as the MalE protein of E.
Mammalian cells are generally more difficult to work with than bacteria since they are much more fragile often produce less product overall and can be much more expensive. To overcome these chromatin effects we have employed a Bacterial. Bacteria lack the machinery for making post-translational modifications.
Answer to Solved Recombinant proteins produced by Ecoli cells are not. When the protein of interest cannot be detected through a sensitive technique eg Western blot or it is detected but at very low levels less than micrograms per liter of culture the problem often lies in a harmful effect that the heterologous protein exerts on the cell Miroux and Walker 1996. This lack of organelles is where bacteria begin to lose their advantage especially when it comes to studying mammalian proteins.
Escherichia coli is the most popular bacterial host for recombinant proteins production due to. However multi-domain eukaryotic proteins expressed in bacteria often are non-functional because the cells are not equipped to accomplish the required post-translational modifications or molecular folding. 6 Why are bacteria good choice for genetic engineering.
Bacterial cells often cannot produce recombinant proteins that are identical to their wild type. Because eukaryotic promoters do not work in bacterial cells it is necessary to provide a bacterial promoter. Minimal post-translational modifications in bacteria Ecoli can not glycosylate proteins which is an extremely common post-translational modification of eukaryotic proteins The protein may not fold into the correct tertiary structure and inclusion bodies may form within the bacteria.
1 its fast growth rate with a generation time spanning 20 min under optimized conditions Clark and Maaløe 1967 2 well-developed tools of molecular manipulations along with in-depth knowledge of its biology and 3 the ability to achieve high cell density using. One feature of the T7 system is that many recombinant proteins often precipitate when expressed at 37 C but are soluble when the temperature during induction is 1525 C presumably because slower rates of protein production allow newly transcribed recombinant proteins time to fold properly 50. 2 Why are bacterial cells useful in recombinant DNA technology.
In addition bacterial cells cannot carry out the post-translation modiÞcations that are. Although eukaryotic cells can be used to express eukaryotic proteins bacteria are simpler to grow and manipulate genetically. The bacteria may process the recombinant protein incorrectly.
A are harder to culture b lack the machinery for making post-translational modifications. Gram-positive bacteria do not have an outer membrane. 5 Why are bacteria excellent hosts for recombinant DNA experiments.
A Nutrient broth to which no antibiotic has been added. Bacterial protein expression systems are popular because bacteria are easy to culture grow fast and produce high yields of recombinant protein. 47When bacteria are genetically modified to produce a protein product technical difficulties arise because that product is not always secreted from the cell.
The use of conventional expression vectors to obtain cell lines is a cumbersome procedure. Based on the mass spectrometry results the masses of rhEPO expressed in CHO and E. Select the appropriate condition to determine if the plasmid has entered the E-coli bacterial cell.
B Water to which ampicillin has been added. May have less than ideal aa. C Nutrient broth to which ampicillin has been added.
One reason for this is their different cellular structure. Bacteria can express large amounts of recombinant protein but the expressed proteins sometimes do not fold properly. Bacterial expression systems due to their simplicity are often not able to produce a recombinant human protein identical to the naturally occurring wild type.
The production of recombinant proteins is crucial for both the development of new protein drugs and the structural determination of drug targets. At N-terminus or have internal destabilizing sequences 4improper post-translational modification. 3 What is the role of bacteria in genetic engineering recombinant DNA technology.
These problems are due to silencing of randomly integrated expression vectors by the surrounding chromatin. 28 The Open Veterinary Science Journal 2009 3 28-34 1874-318809 2009 Bentham Open Open Access So you Need a Protein - A Guide to the Production of Recombinant Proteins Ramamoorthi Jayaraj1 and Peter M. Therefore it is often desirable to express eukaryotic proteins in bacteria Fig.
Fungi plant to synthesize recombinant proteins.
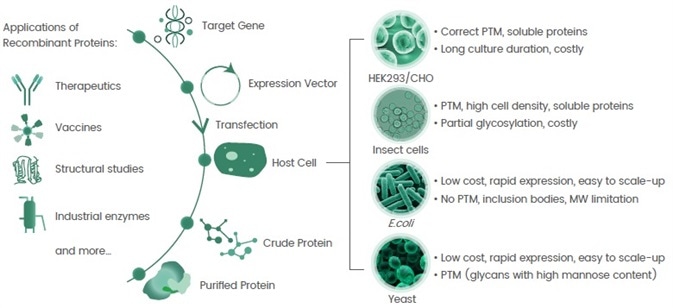
The Challenges Faced In Recombinant Protein Expression
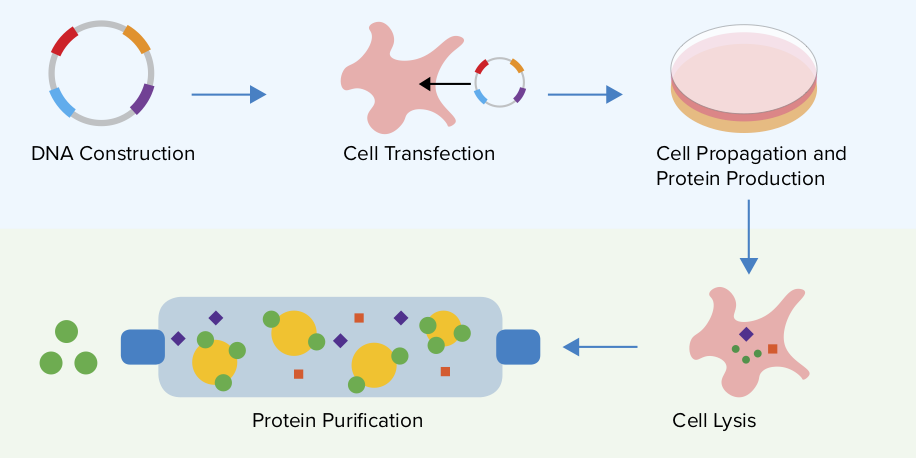
Plasmids 101 Protein Expression

Ijms Free Full Text Escherichia Coli Extract Based Cell Free Expression System As An Alternative For Difficult To Obtain Protein Biosynthesis Html

No comments for "Bacterial Cells Often Cannot Produce Recombinant Proteins"
Post a Comment